

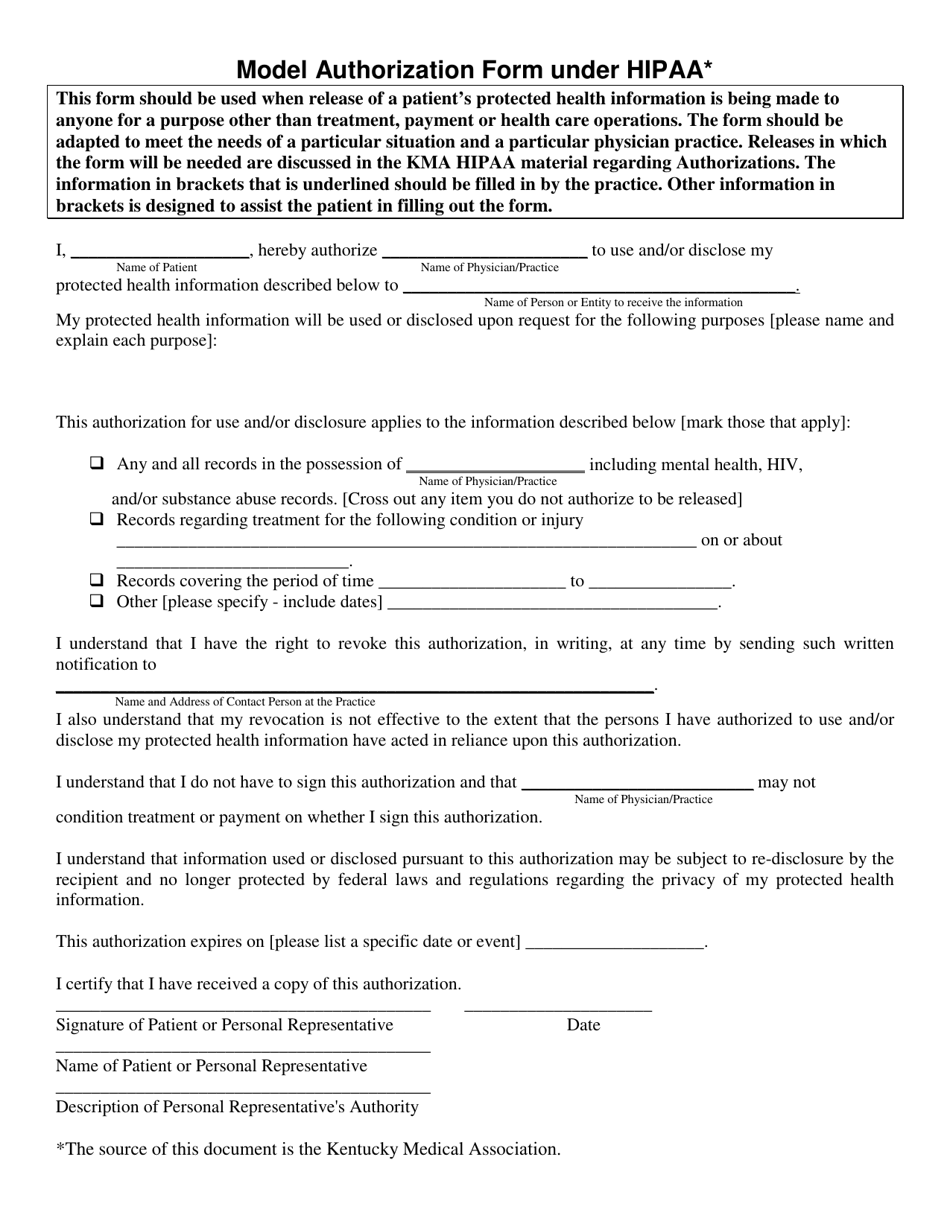
is created or received by a health care provider, health plan, public health authority, employer, life insurer, school or university, or health care clearinghouse and.Health Information : any information, whether oral or recorded in any form or medium, that:.Disclosure: release, transfer, provision of access to, or divulging in any other manner of PHI outside the entity holding the information.A record is any item, collection, or grouping of information that includes PHI and is maintained, collected, used, or disseminated by or for a covered entity. Designated Record Set : group of records maintained by or for a covered entity that includes (1) medical and billing records about individuals maintained by or for a covered health care provider (2) enrollment, payment, claims adjudication, and case or medical management record systems maintained by or for a health plan or (3) used, in whole or in part, by or for the covered entity to make decisions about individuals.Data Use Agreement: agreement into which the covered entity enters with the intended recipient of a limited data set that establishes the ways in which the information in the limited data set may be used and how it will be protected.Covered Entity: health plan, a health care clearinghouse, or a health care provider transmitting health information in electronic form in connection with a transaction subject to the HIPAA regulations.Except as otherwise permitted by the Rule, a covered entity may not use or disclose PHI for research purposes without a valid Authorization. Authorization: An individual’s written permission to allow a covered entity to use or disclose specified PHI for a particular purpose.Refer to UIC policy Research Data Security for a description of UIC policies and procedures related to protecting and securing research data, including PHI.In addition to the HIPAA privacy rule, UIC IRBs apply existing federal regulations, state laws and UIC policies governing human subject research and protecting subject privacy and confidentiality of their private information when reviewing research involving PHI.If the information is released in the form of a limited data set, with certain identifiers removed, and with a data use agreement between the researcher and the covered entity.If research on decedent’s information or.If the PHI has been de-identified in accordance with the standards set by HIPAA.If the IRB has granted a waiver of the authorization requirement.If the individual to whom the PHI belongs has granted specific written permission through an authorization.HIPAA permits the use or disclosure of PHI for research under the following circumstances and conditions:.creating new medical records because as part of the research a health-care service is being performed, such as testing of a new way of diagnosing a health condition or a new drug or device for treating a health condition.deriving research information by retrospectively or prospectively reviewing medical records.The UIC Institutional Review Boards (IRBs) apply the provisions of the Health Insurance Portability and Accountability Act of 1996 and Omnibus Final Rule of 2013 when reviewing research that creates, uses or discloses PHI.Protected health information obtained or maintained by covered components of University of Illinois at Chicago for research purposes may not be used internally or disclosed to any persons or organizations outside the Covered Component for research purposes without prior review and approval of the UIC IRB.This policy applies to any UIC investigators and research staff requesting to create, access or use for research purposes any protected health information (PHI) obtained or maintained by the covered components of the University of Illinois at Chicago (UIC).


 0 kommentar(er)
0 kommentar(er)
